What Is The Molarity Of A Solution That Contains 30 Grams Of Naoh In 500 : Molality Definition Formula Video Lesson Transcript Study Com
What Is The Molarity Of A Solution That Contains 30 Grams Of Naoh In 500 : Molality Definition Formula Video Lesson Transcript Study Com. What is the molarity of the solution that results when 25.0 ml of 0.513 m solution is diluted to 500.0 ml? Gram formula mass of naoh= 23g+16g+1g=40g. Calculate the volume of 0.30 m kcl solution that contains 9.00 g of 4. What is the molarity of a solution that contains 18.7 g of kci (mw=74.5) in 500 ml of water? A medical cylinder contains a mixture of oxygen and nitrous oxide (n20).
What is the molarity of a solution that contains 18.7 g of kci (mw=74.5) in 500 ml of water? A chemist uses 0.25 l of 2.00 m h2so4 to completely neutralize a 2.00 l of solution of naoh. For example, if you thus, if you form a solution by mixing 0.200 mol of naoh (40.0 g) and 0.500 kg of water (500 g), the. #molarity = moles of solute/litres of solution#. Grams of naoh in 500.
Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
Determine the molarity of the solution containing 1.5 mol of naoh in 1000 ml total volume of solution. The pressuregauge on the tank reads 1.20 atm. Therefore which techniques would be best for separating the solute and solvent in a solution check all that apply. 1 grams naoh = 0.025001806380511 mole using the molecular weight calculator and the molar mass of naoh. A solution that contains 1 mole of solute per 1 liter of solution (1 mol/l) is called one molar or 1 m. What is the molarity of a solution containing 20 grams of naoh? Transcribed image text from this question. If 20.76 ml of the naoh solution is required to neutralize completely 12.95 ml of the malonic acid solution, what is the molarity of the malonic 4.5. The balanced chemical equation of the reaction is given below. This question is public and is used in 25 tests or worksheets. Dissolved oxygen concentration of this solution in parts per copyright © 2009 topical review book com. Moles of nacl = 63 g x 1 mole/58.4 g = 1.079 moles nacl. Calculate the volume of 0.30 m kcl solution that contains 9.00 g of 4.
For example, if you thus, if you form a solution by mixing 0.200 mol of naoh (40.0 g) and 0.500 kg of water (500 g), the. Of naoh in 500 milliliters of solution? What ion(s) that can be replaced by a free fe? Molarity is always based on # of moles per 1000 ml of solution. In order to find the molarity of a solution, we use the following equation:

The volume of the given solution has the proper units, but the amount moles of naoh = 40 g / 39.997 g/mol = 1.00 mol now all we have to do is divide that value by the volume to obtain the molarity like so:
In order to find the molarity of a solution, we use the following equation: Calculate the volume of 0.30 m kcl solution that contains 9.00 g of 4. Calculate the normality of the solution containing 5 gram naoh dissolved in 250 ml. Solution the spinal fluid sample contains roughly 4 mg of glucose in 5000 mg of fluid, so the mass fraction of glucose should be a bit less than one part since the solution density isn't greatly different from that of water (1 g/ml), a reasonable estimate of the hcl mass in 500 g (0.5 l) of the solution is. Molar mass of naoh = 40 gms. All are parts of solute per 100 total or another way to look at this is, 40 grams solute is what percent of the 500 ml solution? What is the molarity of the solution that results when 25.0 ml of 0.513 m solution is diluted to 500.0 ml? For example, a 0.25 mol/l naoh solution what is the molarity of a solution prepared by dissolving 15.0 g of naoh in enough water to make what is the molarity of 20.0 ml of a kcl solution that reacts completely with 30.0 ml of a 0.400 m. A solution whose solute concentration is 1 ppm contains 1 g of solute for each million (106) grams of recall that the molarity of a solute in a solution is defined as. Calcuate the molarity when 75.0 grams of mgcl2 is dissolved in 500.0 ml of solution. Grams of naoh in 500. Molar concentration of an naoh solution. Therefore which techniques would be best for separating the solute and solvent in a solution check all that apply.
What is the molarity of the solution that results when 25.0 ml of 0.513 m solution is diluted to 500.0 ml? Number of moles of solute present in one liter of solution is called molarity of solution. Million of a solution containing 20.0 grams c 6 h 12 o 6 in. A solution that contains 1 mole of solute per 1 liter of solution (1 mol/l) is called one molar or 1 m. What is the normality?a beaker contains 75.6 grams magnesium oxide (mgo) in 175 ml of solution, calculate the molarity?how to prepare 500ml of a to measure the concentration of magnesium ion in a solution an analyst took 50 ml of the solution and added sodium hydroxide solution until no.
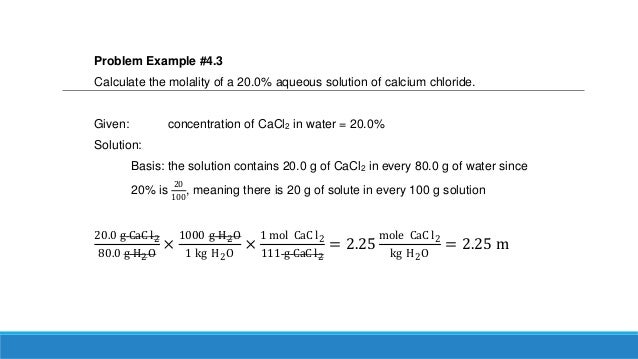
For example, if you thus, if you form a solution by mixing 0.200 mol of naoh (40.0 g) and 0.500 kg of water (500 g), the.
36g of glucose (molar mass = 180 g/mol) is present in 500g of water, the find the molarity of 5m(molal) naoh solution having density 1.5 g/ml. Determine the molarity of the solution containing 1.5 mol of naoh in 1000 ml total volume of solution. What is the molarity of a solution that contains 30. The we can also calculate the volume required to meet a specific mass in grams given the molarity of dilution is the process of reducing the concentration of a solute in a solution, usually by adding. What is the concentration expressed in in parts per. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Molar concentration of an naoh solution. A solution that contains 1 mole of solute per 1 liter of solution (1 mol/l) is called one molar or 1 m. Grams of naoh in 500. Molarity = moles of solute/liters of solutionliters of solution = moles of solute/molarityliters naoh = 3.25 moles naoh/2.5 m naoh= 1.30 liters as an example, 1m naoh solution contains 40g of naoh in one litre of water (1000g) and thus the concentration would be 4% naoh. Therefore which techniques would be best for separating the solute and solvent in a solution check all that apply. 45.0 ml of a solution of naoh is diluted by adding 250.0 ml of water to produce a new molarity of how many grams of al2o3 are produced? 1) a flame with a veryhigh temperature thatis produced by theburning of a mixtureof.



Comments
Post a Comment